
Inspection Readiness - TMF Prep Essential
When an inspection is declared for a study in your Trial Master File (TMF), thorough preparation is key to demonstrating compliance and operational excellence.
Read More →
When an inspection is declared for a study in your Trial Master File (TMF), thorough preparation is key to demonstrating compliance and operational excellence.
Read More →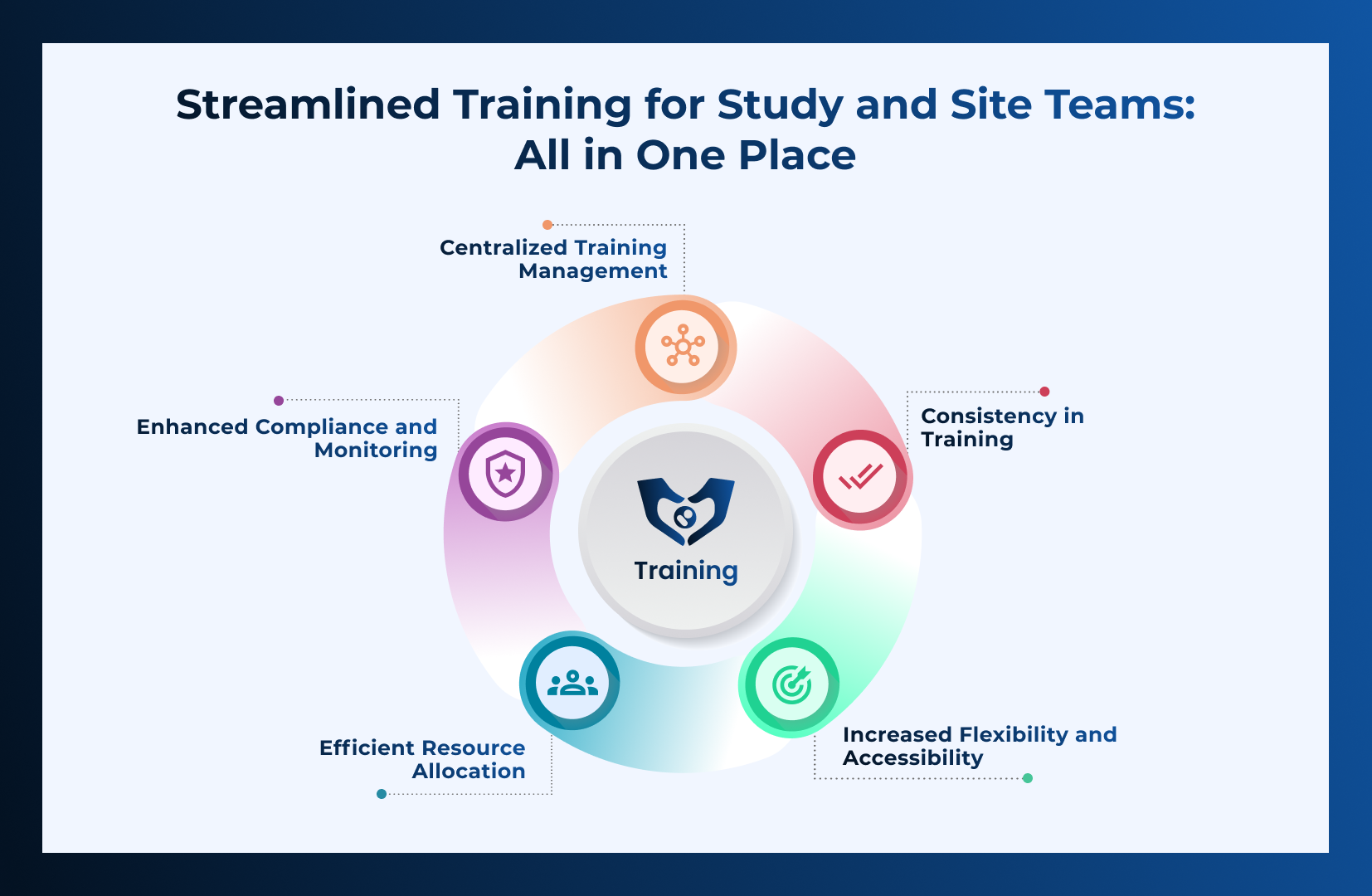
Compliance with regulatory requirements is paramount, in the world of Clinical Resarch. Organizations must adhere to guidelines set by bodies such as the US
Read More →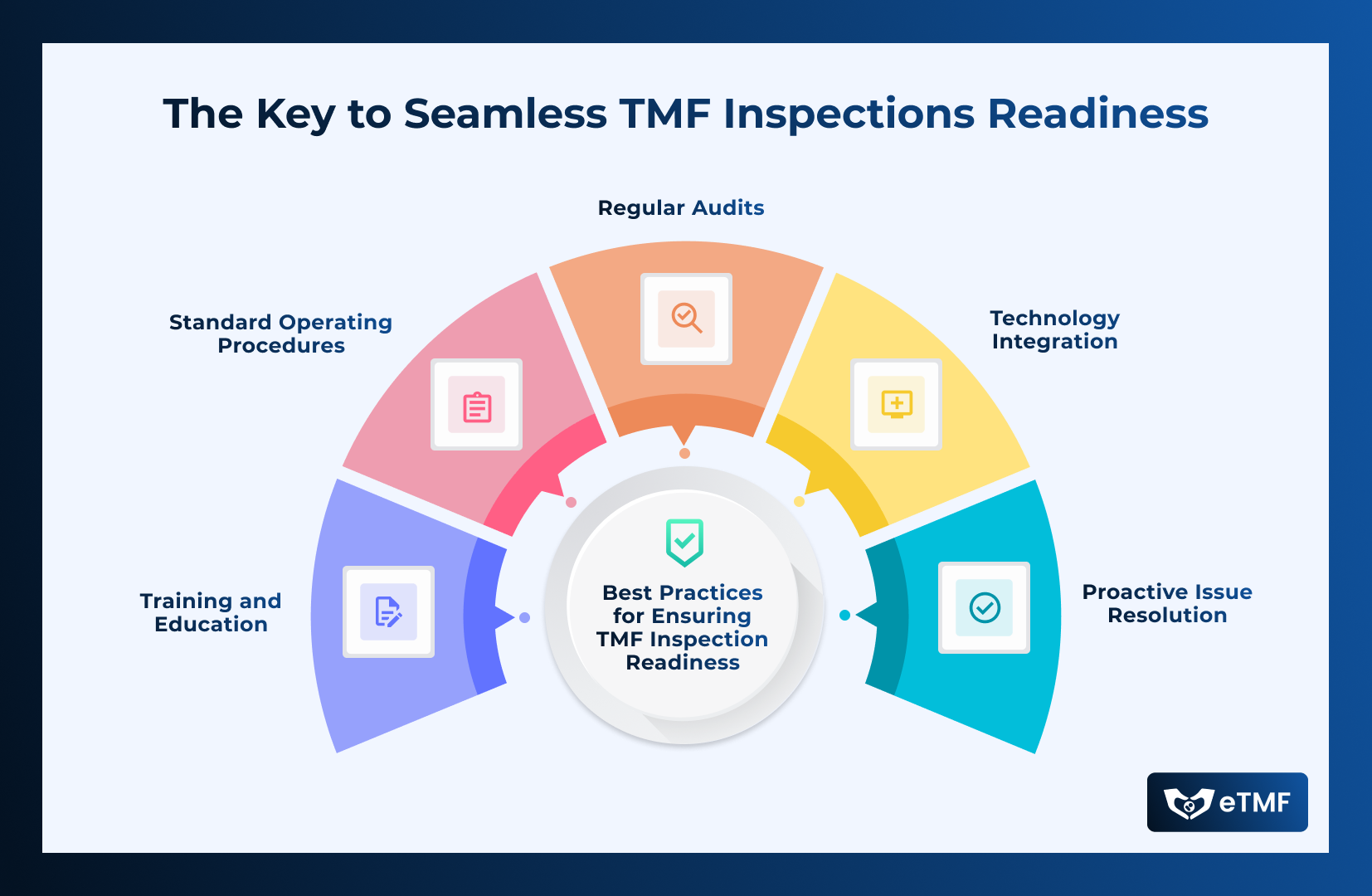
The Trial Master File (TMF) serves as a comprehensive collection of essential documents that chronicle the planning, execution, and results of a study. Ensuring
Read More →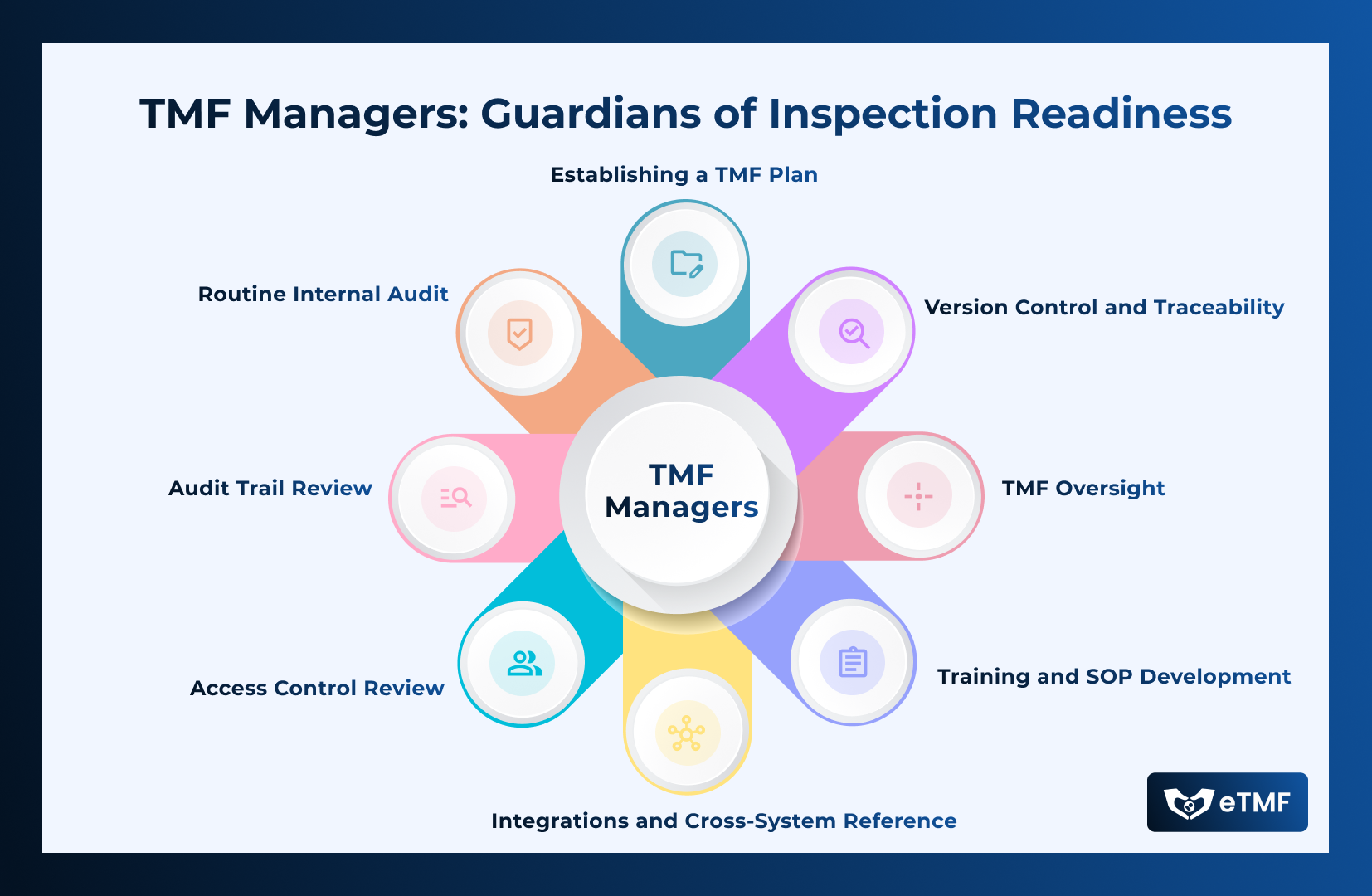
The Trial Master File (TMF) is a critical component in clinical trials, serving as the repository for all essential documents. TMF demonstrate the compliance
Read More →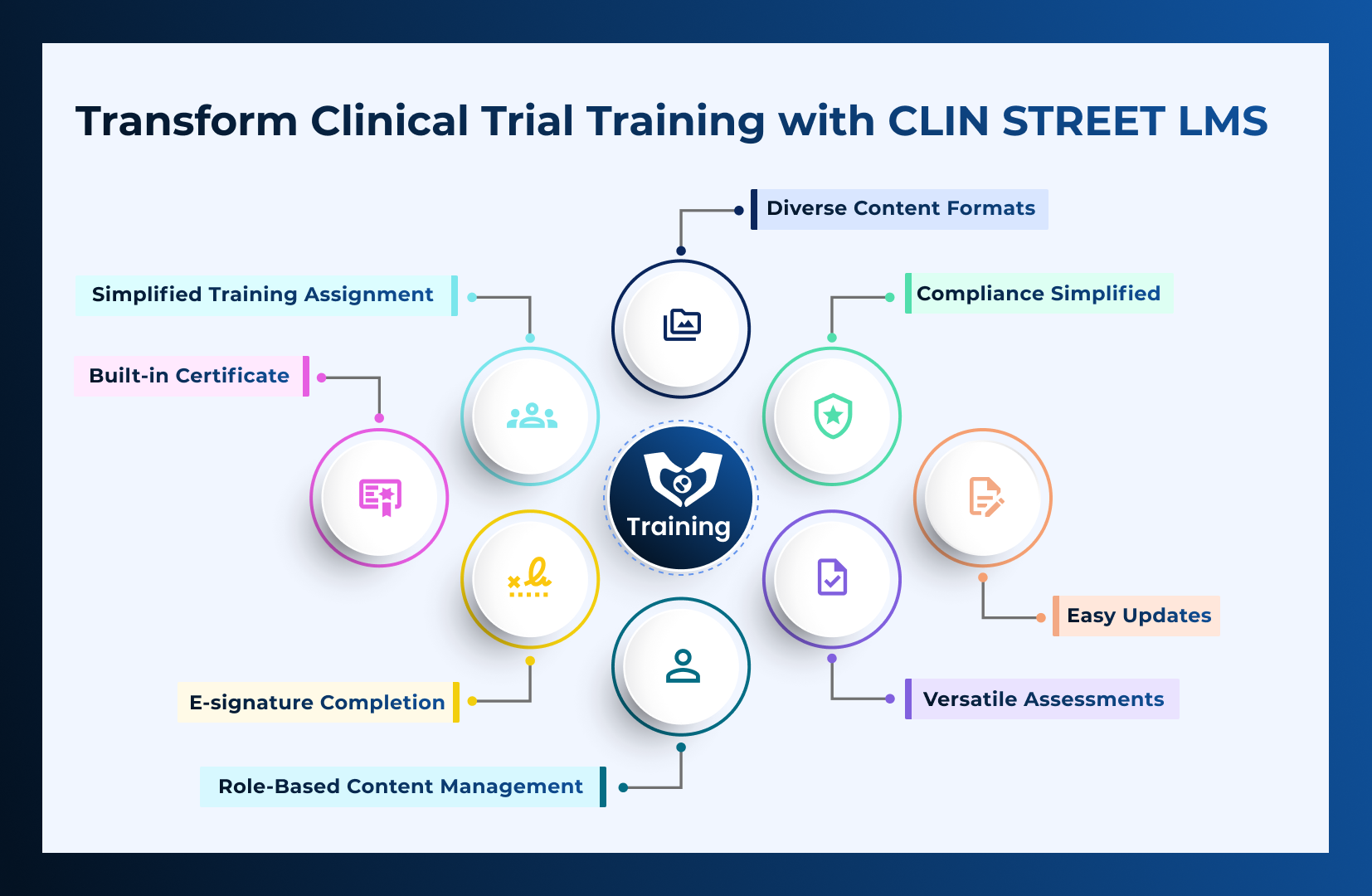
CLIN STREET LMS redefines training! It’s sleek, efficient, and packed with features to make administration a breeze. With a user-friendly interface and powerful tools,
Read More →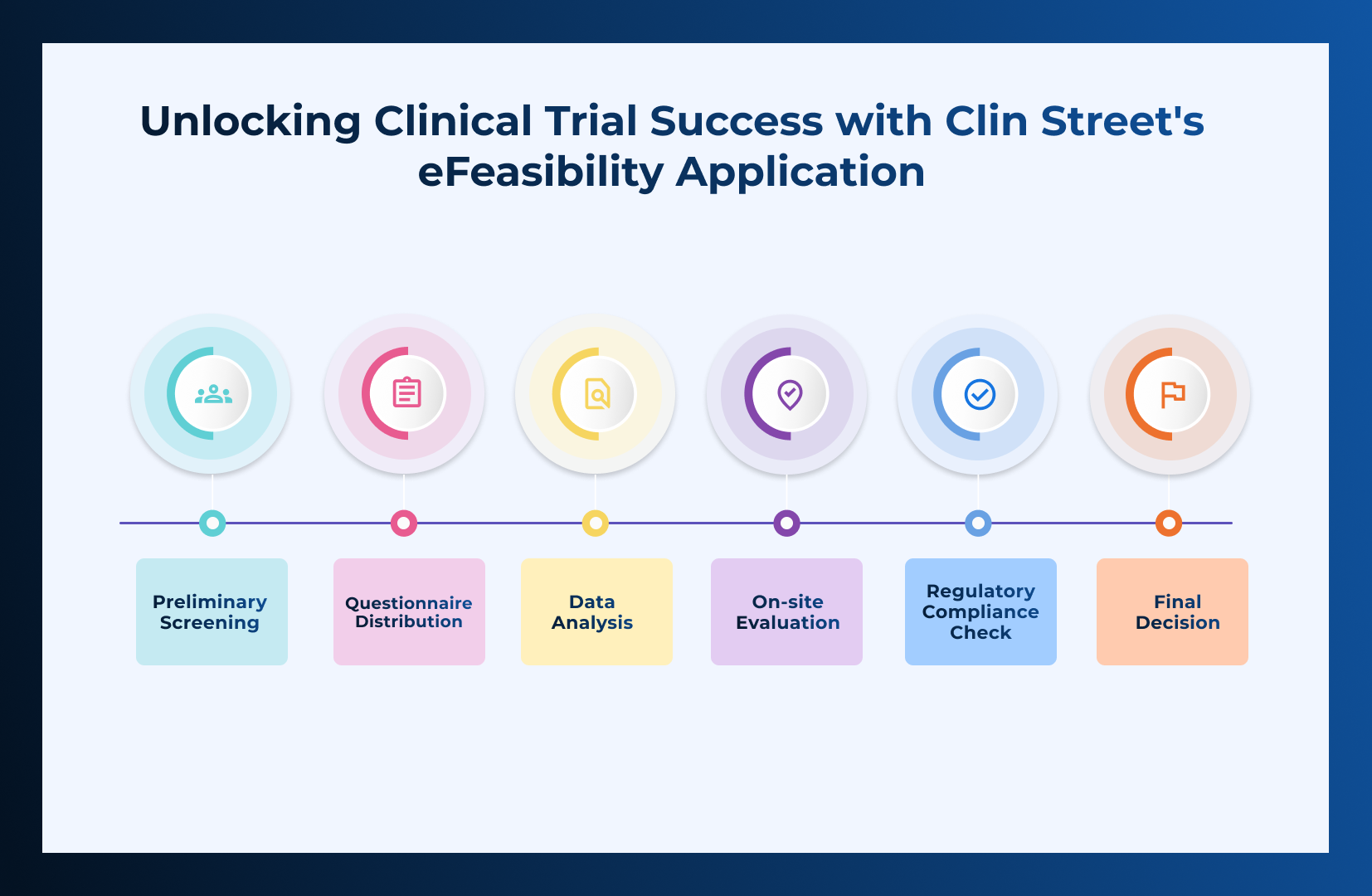
Ensuring that a site is capable of conducting a clinical trial is a pivotal first step in the landscape of Clinical Research!
Read More →