Streamlined Training for Study and Site Teams: All in One Place
By Ashish Sahni |
Compliance with regulatory requirements is paramount, in the world of Clinical Resarch. Organizations must
adhere to guidelines set by bodies such as the US FDA, ICH GCP, and NIH to ensure the safety, efficacy, and
reliability of their studies. A robust Learning Management System (LMS) like CLIN STREET is an invaluable
tool for meeting these standards. Let’s explore the regulatory landscape, the benefits of a unified training
platform, and how to choose the best training applications.
Regulatory Requirements for Training Compliance
- US FDA 21 CFR Part 812.43 : A sponsor shall select investigators qualified by training and experience to investigate the device.
- ICH GCP E6 R3 2.1.2 : The investigator should be familiar with the appropriate use of the investigational product(s) as described in the protocol, in the current Investigator’s Brochure, in the product information and/or in other information sources provided by the sponsor.
- NIH Clinical Research Site Personnel Qualifications, Training and Responsibilities Version 3.0 – 2 October 2023 : The PI/IoR must maintain current and complete training records and make them available to DAIDS, DAIDS representatives, and inspectors, as requested or during site Visits
- NIH Clinical Research Site Personnel Qualifications, Training and Responsibilities Version 3.0 – 2 October 2023 : The CRS personnel should have access to and be trained on protocol and related documents, such as study-specific procedures, Letters of Amendments, Clarification Memos, Manuals of Operations/Procedures, study product specifics, Study Specific Procedures, any specific laboratory procedures, Case Report Form completion guidelines, and any other applicable documentation required to conduct their duties
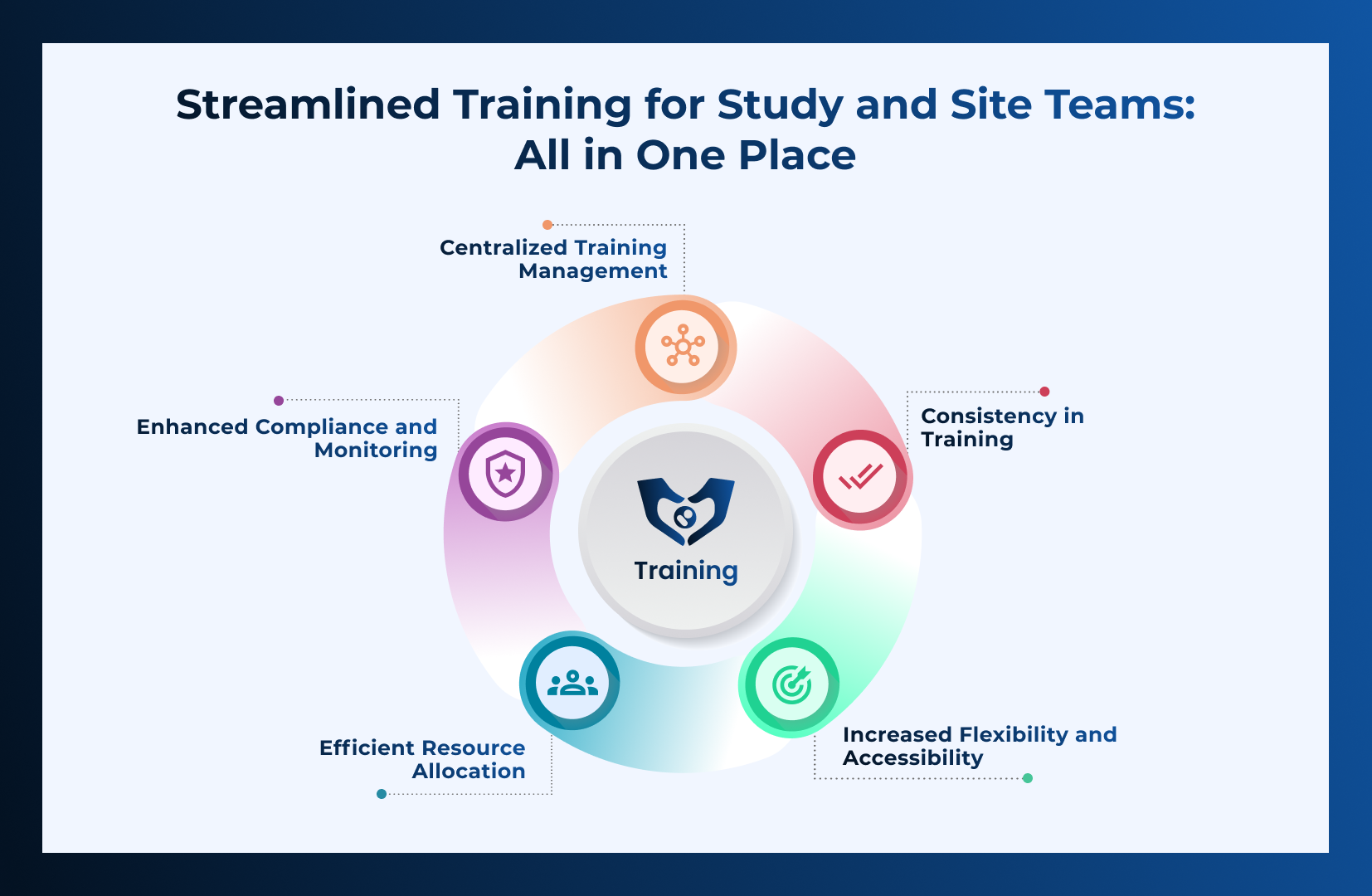
Advantages of a Unified Training Platform
Utilizing a single training platform like CLIN STREET offers significant benefits for all stakeholders
involved in clinical research:
- Centralized Training Management : A unified LMS ensures that all training materials and records are stored in one location. This centralization simplifies tracking, reporting, and auditing processes, ensuring regulatory compliance across the board.
- Consistency in Training : Having a single platform guarantees uniformity in training content and delivery, which is crucial for maintaining standard procedures and practices across diverse teams and locations.
- Increased Flexibility and Accessibility : A unified LMS offers increased flexibility and accessibility for study and site teams. Training modules can be accessed online from anywhere at any time, accommodating the diverse schedules and locations of clinical research staff. This ensures that all team members can complete their training at their own pace, leading to better retention and understanding of the material.
- Efficient Resource Allocation : By consolidating training resources, organizations can reduce redundancy, lower costs, and ensure that all stakeholders have access to the same high-quality training materials.
- Enhanced Compliance and Monitoring : A robust LMS facilitates ongoing monitoring of training progress and compliance, making it easier to identify gaps and ensure that all personnel are up-to-date with their required training.
Choosing the Best Training Applications
When selecting a training application, consider the following criteria to ensure it meets the needs of your
clinical research organization:
- User-Friendly Interface : The application should be intuitive and easy to navigate for users at all levels of technical proficiency.
- Customizability : Look for platforms that allow customization of training modules to fit specific regulatory requirements and organizational needs.
- Scalability : The LMS should be scalable to accommodate the growth of your organization and the increasing complexity of clinical trials.
- Robust Reporting and Analytics : Effective reporting tools are crucial for tracking progress, identifying compliance gaps, and generating audit-ready reports.
- Integration Capabilities : Choose a platform that integrates seamlessly with other systems used in clinical trials, such as Electronic Data Capture (EDC) systems, Safety Databases, and Trial Master Files (TMF).
- Support and Training : Ensure that the vendor provides comprehensive support and training for the LMS to facilitate smooth implementation and ongoing use.
- Security and Compliance : The application must comply with data protection regulations such as GDPR and offer robust security features to protect sensitive information.
Incorporating a comprehensive LMS like CLIN STREET is essential for meeting regulatory requirements and
ensuring the smooth operation of clinical trials. By leveraging a unified training platform, organizations
can enhance compliance, improve efficiency, and foster better communication among all stakeholders involved.
When selecting an LMS, consider factors such as usability, customizability, scalability, and integration
capabilities to find the best fit for your organization’s needs. With the right tools and approach, clinical
research organizations can achieve excellence in training and compliance, ultimately contributing to the
success of their clinical trials.
CLIN STREET’s LMS platform offers a comprehensive suite of features designed to ensure best in class training management for all stakeholders. Key features include:
- Immediate Deployment – Start benefiting from the system on Day 1.
- Versatile Content Support – Easily manage documents, slides, and videos in one platform.
- Training Verification: Conduct knowledge assessments and obtain eSignatures.
- Consistent Quality Assurance: Built-in versioning and quality workflows.
- Flexible Task Assignment: Assign tasks by role or individual.
- User-Friendly Interface: Intuitive UI requires minimal training.
- Automated Certification: Generate certificates automatically.
- Accessibility: Responsive and mobile-friendly.
- Enhanced Accountability: Comprehensive audit and activity logs.
- Inclusive Access: Secure login for all stakeholders to collaborate efficiently.
We understand that achieving Compliance for Training can be delicate and your needs are unique. That’s why
we’re here to offer a tailor-made walkthrough of our CLIN STREET Training platform, designed to simplify and
enhance your clinical trial management process. Let’s explore together how our solutions can be precisely
aligned with your project’s requirements by scheduling a personalized demonstration here. Alternatively, you can also contact
us at hello@clinstreet.com. Our team is available around the clock to accommodate your schedule and provide
the support you need