The Key to Seamless TMF Inspection Readiness
By Ashish Sahni |
The Trial Master File (TMF) serves as a comprehensive collection of essential documents that chronicle the
planning, execution, and results of a study. Ensuring TMF inspection readiness is not just a regulatory
necessity but a cornerstone of credible and reliable clinical research. This blog delves into the importance
of TMF inspection readiness, its impact on regulatory approval, and best practices for maintaining a
compliant TMF.
The Role of TMF in Clinical Trials
- Central Repository: The TMF acts as the central repository for all trial-related documents, ensuring that every aspect of the clinical trial is meticulously documented.
- Regulatory Compliance: It demonstrates compliance with regulatory requirements, such as ICH GCP guidelines, FDA regulations, and EMA standards, which are crucial for the approval of new drugs and therapies.
- Data Integrity: A well-maintained TMF ensures the integrity and reliability of trial data, providing a clear and accurate trail of all activities conducted during the study.
Why TMF Inspection Readiness Matters
- Regulatory Approval: Regulatory authorities scrutinize the TMF during inspections to assess compliance with clinical trial regulations. A disorganized or incomplete TMF can lead to delays or denials in regulatory approval.
- Credibility and Trust: An inspection-ready TMF enhances the credibility of the clinical trial, building trust with regulators, sponsors, and stakeholders.
- Risk Mitigation: Regularly maintaining an inspection-ready TMF helps identify and mitigate risks associated with non-compliance, such as financial penalties, legal actions, and reputational damage.
- Efficiency and Quality: An organized TMF improves operational efficiency and ensures high-quality data management, reducing the likelihood of errors and discrepancies.
Key Components of TMF Inspection Readiness
- Comprehensive Documentation: Ensure all required documents are present, complete, and up-to-date. This includes study protocols, informed consent forms, monitoring reports, correspondence, and regulatory approvals.
- Organization and Accessibility: Maintain a well-organized TMF with clear indexing and easy accessibility to all documents. This facilitates quick retrieval during inspections.
- Version Control: Implement robust version control practices to manage document revisions and ensure that the most current versions are available.
- Audit Trails: Maintain detailed audit trails that document every change made to the TMF, including who made the change, when, and why.
- Quality Control: Conduct regular quality control checks to identify and rectify any issues promptly, ensuring ongoing compliance.
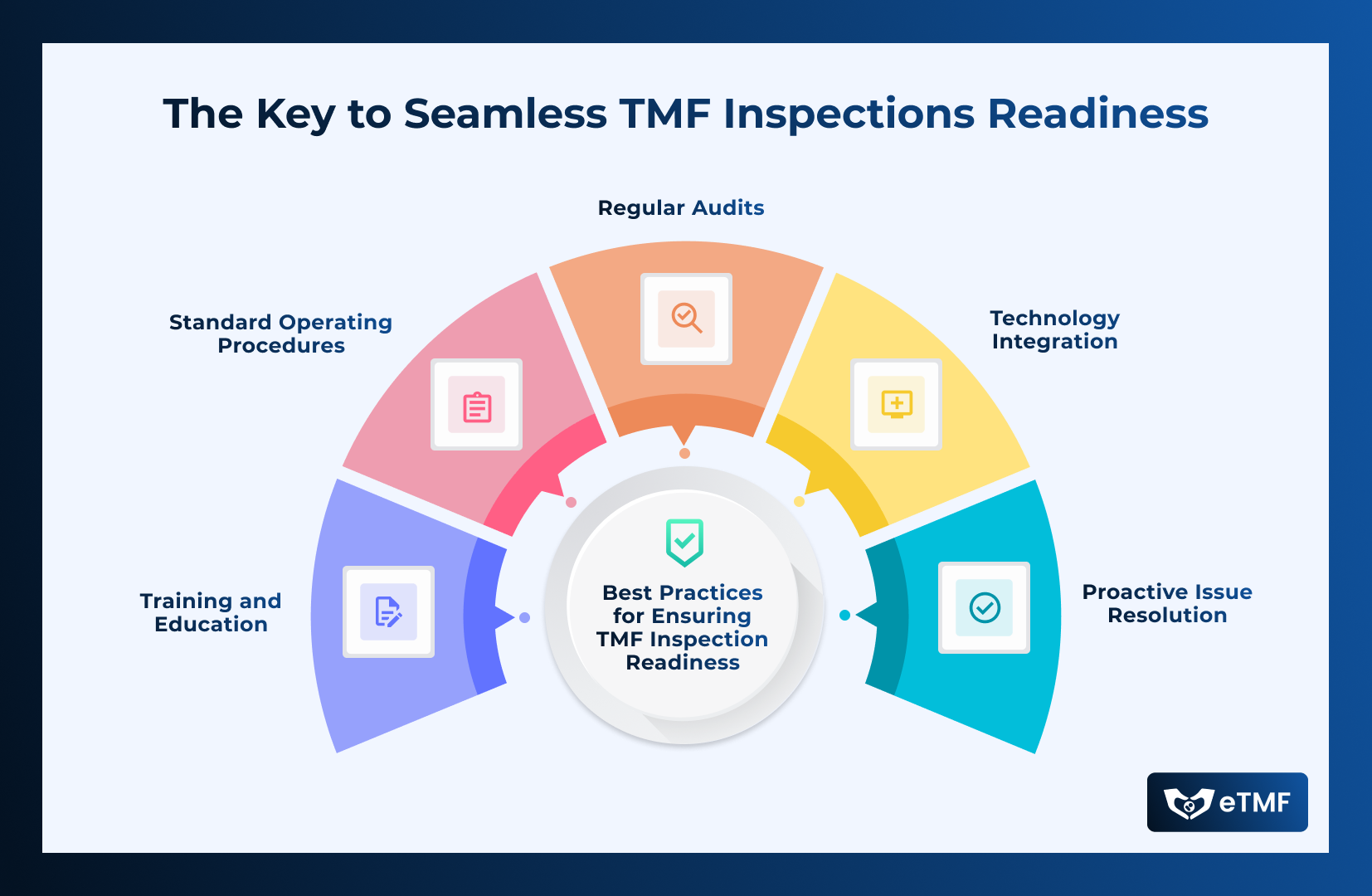
Best Practices for Ensuring TMF Inspection Readiness
- Regular Audits: Conduct routine internal audits to review the completeness and accuracy of the TMF, identifying and addressing any gaps.
- Standard Operating Procedures (SOPs): Develop and adhere to clear SOPs for TMF management, ensuring consistency and compliance in document handling.
- Training and Education: Provide ongoing training for clinical trial staff on TMF management best practices and regulatory requirements.
- Technology Integration: Leverage electronic TMF (eTMF) systems to streamline document management, improve accessibility, and enhance data integrity.
- Proactive Issue Resolution: Address any issues or discrepancies as soon as they are identified, rather than waiting for an external inspection to highlight them.
The importance of TMF inspection readiness in clinical trials cannot be overstated. It is fundamental to
achieving regulatory approval, maintaining data integrity, and ensuring the overall credibility of clinical
research. By implementing best practices and leveraging technology, clinical trial teams can maintain an
inspection-ready TMF, mitigate risks, and streamline the path to successful trial outcomes.
Staying proactive in TMF management not only demonstrates a commitment to regulatory compliance but also
underscores the dedication to conducting high-quality, reliable clinical research.
CLIN STREET’s eTMF platform offers a comprehensive suite of features designed to ensure best in class
management of TMF towards achieving Inspection readiness. Key features include:
- Dashboard : Real-Time Comprehensive dashboards aiding oversight covering all KPIs such as Completeness, Quality, Timeliness.
- Site Level -Dashboards: Visibility of Site level expected documents per site in dashboard view along with oversight on progress of document filing per milestones and events.
- Real-Time Built-In Report : In-depth visibility on reports to assess gaps and derive triggers for improvements
- AutoPilot : First class Built-In automation that elevates the management of milestones and events and drive Trials towards compliance.
- Milestone-Event driven expected document creation : Best in class feature facilitating creation of expected documents in trial, while associating those with milestones and events.
- Milestone-Event Based Document Management: Efficiently collect and assess document completeness in alignment with critical milestones and events, ensuring timely and organized document handling.
- Comprehensive Document Tracking: Seamlessly plan, manage, and monitor all expected documents within the eTMF, ensuring nothing falls through the cracks.
- Direct Site Upload Capability: Simplify the document submission process with direct upload capabilities for sites, enhancing efficiency and reducing delays.
- Integrated Reporting and Notifications: Stay informed with built-in reports and automated notifications, keeping you up-to-date on TMF status and facilitating prompt action when necessary
- Comprehensive Audit Trail: In-detail audit trail giving visibility of each task performed within TMF per user.
- Integration with LMS: Configurable integration with CLIN STREET’s LMS for auto filing of Training certificate, driving compliance of the TMF
Driving Inspection Readiness for TMF can be complex, and we understand your unique needs. That’s why we’re here to introduce you to our CLIN STREET eTMF platform, designed to simplify your clinical trial management. Let’s collaborate to tailor our solutions precisely to fit your project’s requirements by scheduling a personalized demonstration here. Alternatively, you can also contact us at hello@clinstreet.com. Our team is available around the clock to accommodate your schedule and provide the support you need.