Unlocking Clinical Trial Success with CLIN STREET’s eFeasibility Application
By Ashish Sahni |
Ensuring that a site is capable of conducting a clinical trial is a pivotal first step in the landscape of
Clinical Research! CLIN STREET’s eFeasibility application stands at the forefront of this process, offering
a digital solution to streamline and enhance site feasibility assessments. This blog explores the critical
role of site feasibility, the process involved, regulatory observations, and the distinct advantages of
using CLIN STREET’s innovative application.
The Importance of Site Feasibility in Clinical Trials
Site feasibility assessment is a fundamental step in the planning and execution of clinical trials. It
determines whether a site has the necessary resources, infrastructure, and expertise to successfully conduct
a study. A thorough feasibility study mitigates risks, ensures compliance with regulatory standards, and
lays the groundwork for successful trial outcomes.
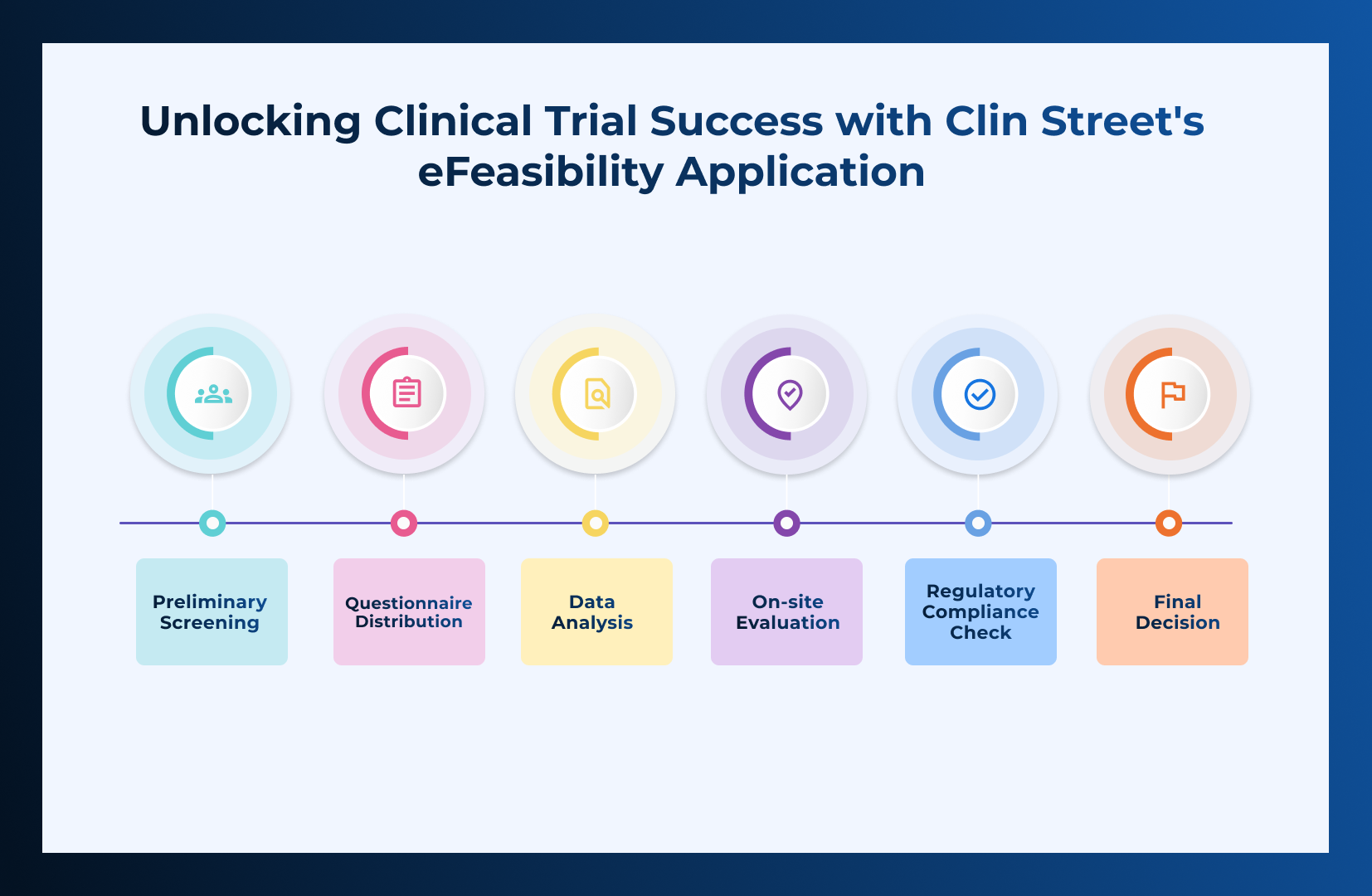
Process of Checking Site Feasibility
- Preliminary Screening: Initial assessment based on basic criteria such as location, patient population, and past performance in clinical trials.
- Questionnaire Distribution: A detailed questionnaire is sent to potential sites, covering aspects like staff qualifications, facility capabilities, previous trial experience, and patient recruitment strategies.
- Data Analysis: Responses are collected and analyzed to evaluate the site’s suitability.
- On-site Evaluation: If necessary, a physical inspection is conducted to verify the provided information and assess the site’s infrastructure and operations.
- Regulatory Compliance Check: Ensuring the site meets all relevant local and international regulatory requirements.
- Final Decision: Based on the collected data and analysis, a decision is made regarding the site’s feasibility.
Regulatory Observations on Site Feasibility
Regulatory authorities, such as the FDA and EMA, have identified some common challenges associated with
sites in being able to run successful trial, these include:
- Inadequate Staffing: Sites often lack personnel with the necessary qualifications and experience.
- Insufficient Patient Population: The site cannot recruit the required number of participants.
- Inadequate Facilities: Sites fail to meet the necessary infrastructure standards, such as laboratory capabilities or storage conditions for investigational products.
- Non-compliance with Good Clinical Practice (GCP): Sites do not adhere to GCP guidelines, risking the integrity and ethical conduct of the trial.
Why Use an Application for Feasibility Studies?
The traditional approach to site feasibility involves extensive paperwork, manual data collection, and
lengthy timelines. Leveraging technology through an application like CLIN STREET’s eFeasibility offers
several key benefits:
- Efficiency: Automates the distribution and collection of questionnaires, reducing time and effort.
- Accuracy: Minimizes human errors in data entry and analysis.
- Real-time Updates: Provides instant updates and feedback, facilitating quicker decision-making.
- Enhanced Collaboration: Enables seamless communication between sponsors and sites.
Advantages of CLIN STREET’s eFeasibility Application
CLIN STREET’s eFeasibility application is designed with user-friendliness and functionality in mind,
offering numerous advantages:
- Intuitive Design: The user interface is straightforward and easy to navigate, reducing the learning curve for users.
- Easy Access: Cloud-based access allows users to complete and review feasibility studies from anywhere, at any time.
- Versatile Questionnaire: Built-In customizable questionnaires tailored to specific trial needs.
- One-click Response Gathering: Collects responses with a single click, streamlining data collection.
- Anonymous Response Collection: Ensures unbiased feedback from sites, leading to more reliable feasibility assessments.
Conducting thorough site feasibility is a critical step in ensuring the success of clinical trials. By
leveraging advanced technology like CLIN STREET’s eFeasibility application, sponsors can streamline the
feasibility assessment process, enhance accuracy, and make informed decisions more rapidly. This innovative
tool not only simplifies the process but also significantly improves the chances of trial success by
ensuring that only the most capable and compliant sites are selected. That’s why we’re here to offer a
tailor-made walkthrough of our CLIN STREET eFeasibility platform, designed to simplify and enhance your
clinical trial management process. Let’s explore together how our solutions can be precisely aligned with
your project’s requirements by scheduling a personalized demonstration here. Alternatively, you can also contact
us at hello@clinstreet.com. Our team is available around the clock to accommodate your schedule and provide
the support you need.