CLIN STREET CTMS
CTMS
Comprehensive Site Solution for Clinical Research - Empower site teams to streamline workflows, maintain compliance, and access all critical data effortlessly.
Powerful Solutions, All In One Platform
Streamline your clinical research operations with our integrated suite of tools designed for maximum efficiency and compliance.
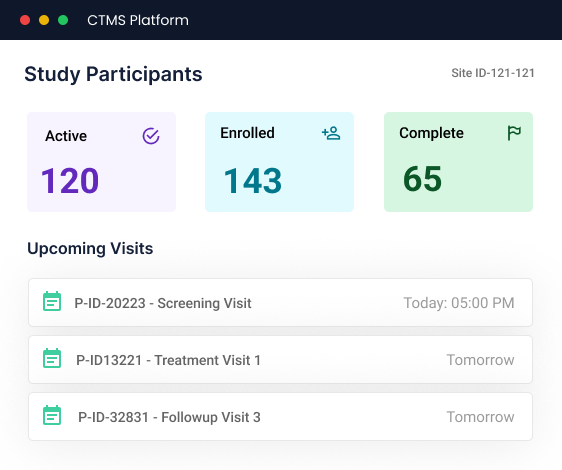
CTMS
Centralized Site Operations for Smarter Trials
- Participant Management
- Compliance & Audit-Readiness
- Financial Workflows
- Reporting and Analytics
eISF
Streamlined Regulatory Binder Management
- Document Version Control
- Automated Audit Trails
- Secure Access Control
- Remote Monitoring
eSource
Digitize and Simplify Data Collection
- Real-Time Data Entry
- Paperless Documentation
- Compliance & Security
- Centralized Data Access
Account Receivables, Simplified
Automate tracking of site payments with integrated account receivables
Automated Tracking
Generate accounts receivable based on participant visits, procedures, and completed milestones — no manual entry required.
Oversight
Easily track and manage outstanding payments by study and sponsor, with full visibility into what’s billed, received, and pending.
Real-Time Status
Access up-to-date insights into the payment lifecycle — from invoice creation to collection — so nothing falls through the cracks.
Predictable Cash Flow
Maintain a clear financial picture across your studies and sites to keep your operations running smoothly and sustainably.
Why Choose CTMS?
Experience the difference with our comprehensive platform designed specifically for clinical research sites.
Streamlined Efficiency
Reduce manual tasks and streamline workflows with automated processes and integrated tools.
Regulatory Compliance
Stay compliant with built-in regulatory features, audit trails, and secure document management.
Time Saving
Save valuable time with automated scheduling, real-time data entry, and centralized access to all information.

Discover How Our Platform Can Simplify Your Clinical Trials