CLIN STREET eTMF
eTMF
Streamline clinical trial document management with our secure, compliant enterprise solution.
Ready on Day-1
Everything you need to get started.
Pre-configured
Our eTMF solution comes equipped with pre-configured, industry-standard templates and workflows, allowing you to hit the ground running. Say goodbye to expensive setups and lengthy configurations – start managing your documents effortlessly from Day-1.
Seamless Compliance
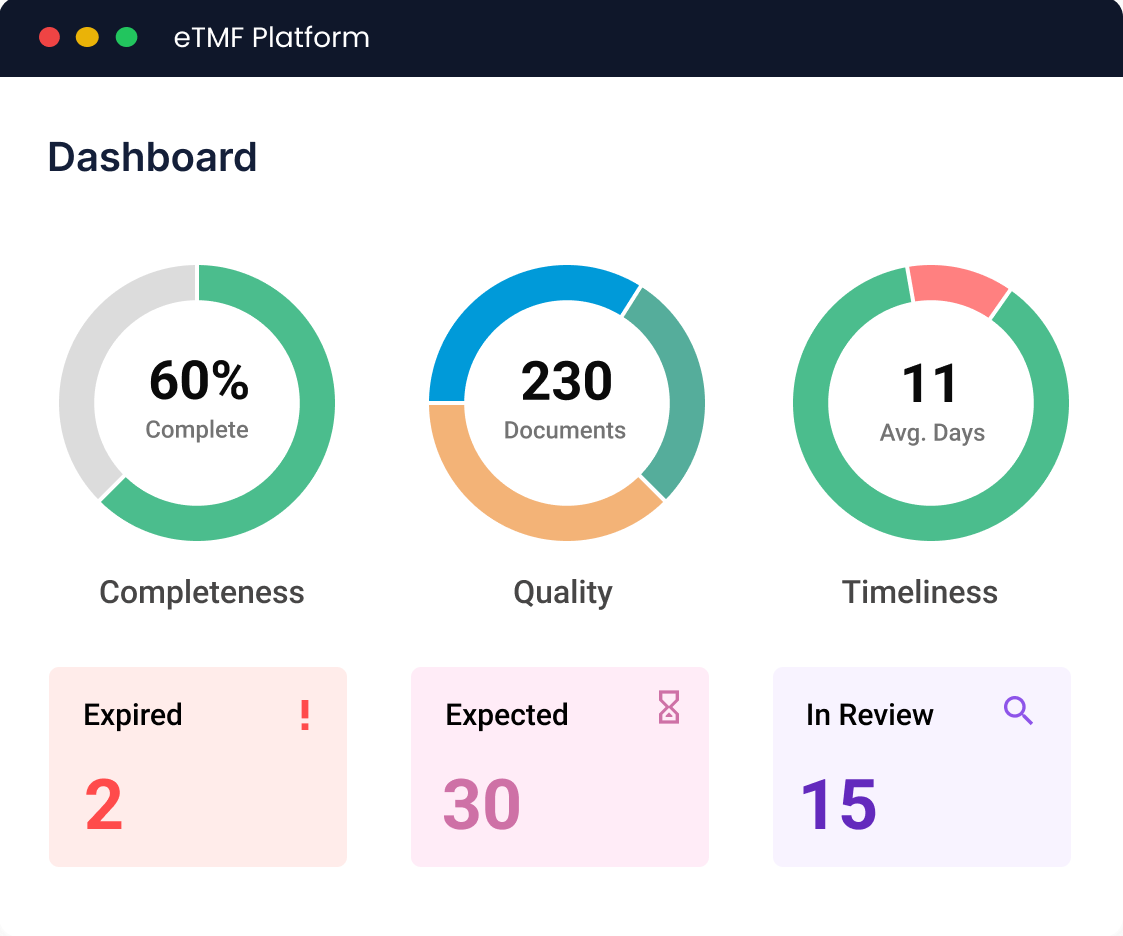
Effortlessly achieve regulatory compliance with our eTMF solution. With built-in quality workflows, expected document tracking, and real-time KPIs, it ensures your documents are systematically organized and securely managed.
Always Audit-Ready
Stay prepared with easy accessibility, real-time tracking of completeness, quality, and timeliness, dynamic reports, and audit dashboard. Enable sites to directly upload documents and ensure seamless readiness for every inspection.
Why Choose CLIN STEET eTMF?
Plan and Manage
- Documents by study and site milestones
- Assign document responsibilities to team members
- Stay informed on document needs based on trial events
- Track document status for timely completion
- Document Analytics to keep document timelines on track
Ready on Day-1
- Minimal training
- Easy to use
- Streamlined workflows
- Effortless document management
- Mobile Friendly User Experience
Analytics and Reporting
- Up-to-date reports for instant trial insights
- Visualize essential metrics to drive informed decisions
- Document completion status at a glance
- Tailored dashboards to highlight critical trial metrics
- Document Analytics to keep document timelines on track
Streamlined eTMF Archival
- Simplify study closure
- Ensure a seamless transition to the archival phase
- Archive essential documents in just days
- Track all documents end-to-end for full visibility
- Maintain comprehensive and compliant record-keeping
Security by Design
21 CFR Part 11 Complaint
Role Based Access Control
Audit Trail and Version History
Support for Unblinded Documents

Discover How Our Platform Can Simplify Your Clinical Trials