In the dynamic world of clinical research, Trial Master Files (TMFs) play a pivotal role in ensuring the integrity, completeness, and compliance of trial documentation. Traditionally, managing TMF milestones has been a labor-intensive process, fraught with challenges such as human error, inefficiencies, and compliance risks. However, the advent of automation technologies is revolutionizing TMF management, offering significant benefits while mitigating compliance risks. Let’s explore the role of automation in managing TMF milestones, its benefits, and the associated compliance considerations.
The Role of Automation in TMF Milestone Management
Automation in TMF milestone management involves leveraging technologies such as artificial intelligence (AI), machine learning (ML), and robotic process automation (RPA) to streamline processes, enhance efficiency, and ensure timely milestone achievement. From document tracking to quality control checks, automation offers a comprehensive solution to the complexities of TMF management.
- Real-Time Progress Evaluation: Automation systems can build capabilities to have real-time evaluation of progress of milestones. It is critical to keep system up to date with respect to milestone due dates and associated expectations. Any deviation from planned date, means forced course correction. Automation systems can be leveraged to not miss these course corrections and keep moving towards inspection readiness of Trial.
- Expected document creation: Every milestone is essentially linked to set of documents that are expected to be created in Trial by various stakeholders. These expected documents can be auto created in huge numbers using automated systems. Having expected document list itself gives confidence about compliance focus in the conduct of the Trial.
- Tracking and Alerts: Automated systems can track TMF milestones in real-time, automatically updating stakeholders on milestone progress and sending alerts for impending deadlines. This proactive approach minimizes delays, improves accountability, and facilitates timely decision-making.
Benefits of Automation in TMF Milestone Management
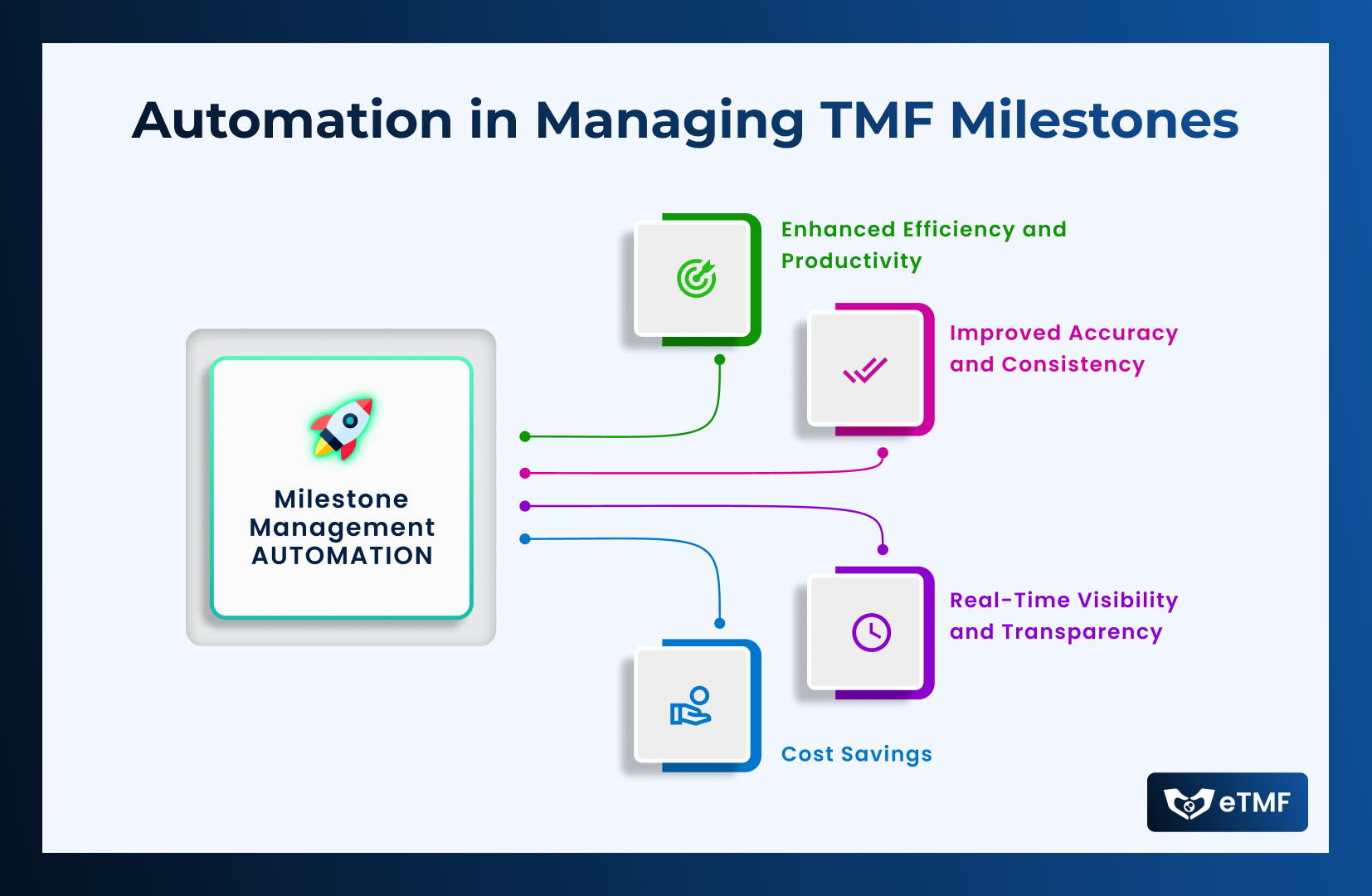
- Enhanced Efficiency and Productivity: By automating repetitive tasks and streamlining processes, automation significantly reduces the time and effort required for TMF milestone management. This allows clinical research teams to focus on more strategic activities, accelerating trial timelines and improving productivity.
- Improved Accuracy and Consistency: Automation minimizes the risk of human error inherent in manual TMF management processes, ensuring greater accuracy and consistency in milestone tracking and document organization. This mitigates the potential for compliance deviations and enhances data integrity.
- Real-Time Visibility and Transparency: Automated TMF systems provide stakeholders with real-time visibility into milestone progress, document status, and compliance metrics. This transparency fosters collaboration, facilitates communication, and enables informed decision-making throughout the trial lifecycle.
- Cost Savings: By reducing manual effort, streamlining processes, and minimizing compliance risks, automation translates into significant cost savings for sponsors and CROs. Moreover, the enhanced efficiency and productivity achieved through automation can lead to faster trial completion and quicker time-to-market, further optimizing resource utilization.
Compliance Risks Associated with TMF without Automation:
If automation is not implemented in Trial Master File (TMF) milestones management, several risks and challenges may arise:
- Increased Errors: Manual processes are prone to human errors such as data entry mistakes, misclassification of documents, and inaccurate milestone tracking. These errors can compromise the integrity and completeness of trial documentation.
- Inefficiency: Manual milestone management is time-consuming and labor-intensive, leading to inefficiencies in document organization, tracking, and reporting. This can result in delays in milestone achievement and overall trial progress.
- Compliance Concerns: Without automated quality control checks and compliance validations, there is a higher risk of overlooking regulatory requirements and documentation standards. This can lead to compliance deviations, regulatory non-compliance, and potential fines or legal repercussions.
- Limited Visibility: Manual processes lack real-time visibility into milestone progress, document status, and compliance metrics. This hampers decision-making, collaboration, and transparency among stakeholders, potentially leading to misunderstandings and delays in addressing issues.
- Resource Constraints: Manual milestone management requires significant human resources, including time, personnel, and expertise. Without automation, organizations may struggle to allocate resources effectively, leading to overburdened staff, increased costs, and reduced productivity.
- Data Security Risks: Manual handling of trial documentation increases the risk of data security breaches, unauthorized access, and data loss. Without proper safeguards and controls, sensitive trial data may be compromised, leading to confidentiality breaches and reputational damage.
- Audit Trail Challenges: Manual processes make it difficult to maintain accurate and comprehensive audit trails of milestone activities. This can hinder regulatory inspections and audits, as well as impede traceability and accountability in the event of compliance investigations.
- Scalability Issues: Manual milestone management may struggle to scale effectively to accommodate growing trial complexities, increased document volumes, and expanding regulatory requirements. This can limit the organization’s ability to manage multiple trials simultaneously and adapt to changing industry dynamics.
- Data Integrity Concerns: Manual data entry and document handling increase the risk of data inconsistencies, duplication, and loss. This compromises data integrity and reliability, undermining the credibility of trial results and hindering scientific advancements.
- Competitive Disadvantage: In today’s fast-paced clinical research environment, organizations that rely solely on manual processes risk falling behind competitors who embrace automation. Without automation, they may struggle to meet evolving industry standards, customer expectations, and regulatory demands, putting their competitive position at risk.
Automation is transforming TMF milestone management, offering unparalleled efficiency, accuracy, and compliance in clinical trial documentation. By leveraging automation technologies, organizations can streamline processes, enhance productivity, and mitigate compliance risks, ultimately accelerating the development of life-saving therapies and improving patient outcomes in clinical research. However, it is imperative to carefully consider and address the compliance implications associated with automation to ensure regulatory adherence and data integrity throughout the trial lifecycle.
CLIN STREET’s eTMF platform offers a comprehensive suite of features designed to ensure best in class milestone management and associated expected documents. Key features include:
- Built-In Milestone Implementation : CLIN STREET’s Built-In Ready-to-Use milestone templates that can be implemented instantly, to get started with management of trial.
- Custom Milestones : Dynamic feature with ability to build multiple templates of milestones that can be implemented across one or more studies in scope, basis requirement.
- Milestone Date Edits : Ability to provision due dates during implementation of milestones and edit dates on run time.
- Milestone driven expected document creation : Best in class feature facilitating creation of expected documents in trial, while associating those with milestones.
- Site Level Dashboards: Visibility of Site level milestones per site in dashboard view along with oversight on progress of document filing per milestone.
- AutoPilot : First class Built-In automation that elevates the management of milestones and drive Trials towards compliance.
Managing TMF milestones in-depth can be complex, and we understand your unique needs. That’s why we’re here to introduce you to our CLIN STREET eTMF platform, designed to simplify your clinical trial management. Let’s collaborate to tailor our solutions precisely to fit your project’s requirements by scheduling a personalized demonstration here. Alternatively, you can also contact us at hello@clinstreet.com. Our team is available around the clock to accommodate your schedule and provide the support you need.
About the Author: Shounak Damle is a seasoned clinical trial professional with extensive experience in TMF management and other eClinical technologies. With a passion for quality assurance and compliance.