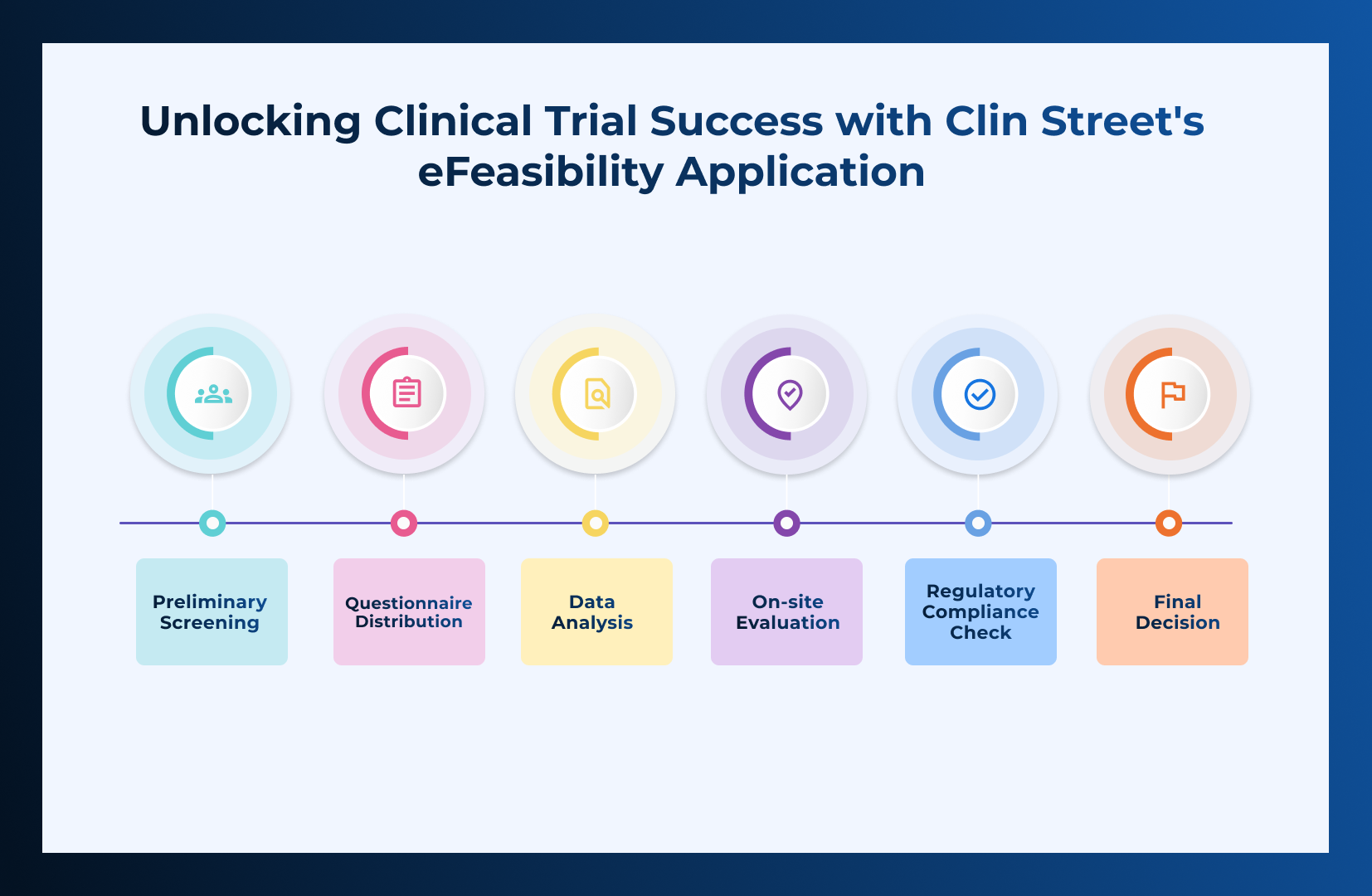
Unlocking Clinical Trial Success with CLIN STREET’s eFeasibility Application
Ensuring that a site is capable of conducting a clinical trial is a pivotal first step in the landscape of Clinical Research!
Read More →ATLAS
Discover trial-ready sites, sponsors and opportunities across the globe with just a click.
Site Solutions
Streamline site operations with integrated eISF, eSource, participant tracking, and financial workflows.
Sponsor Solutions
Design, manage and monitor your studies with powerful and audit-ready study tools.

Discover How Our Platform Can Simplify Your Clinical Trials

Ensuring that a site is capable of conducting a clinical trial is a pivotal first step in the landscape of Clinical Research!
Read More →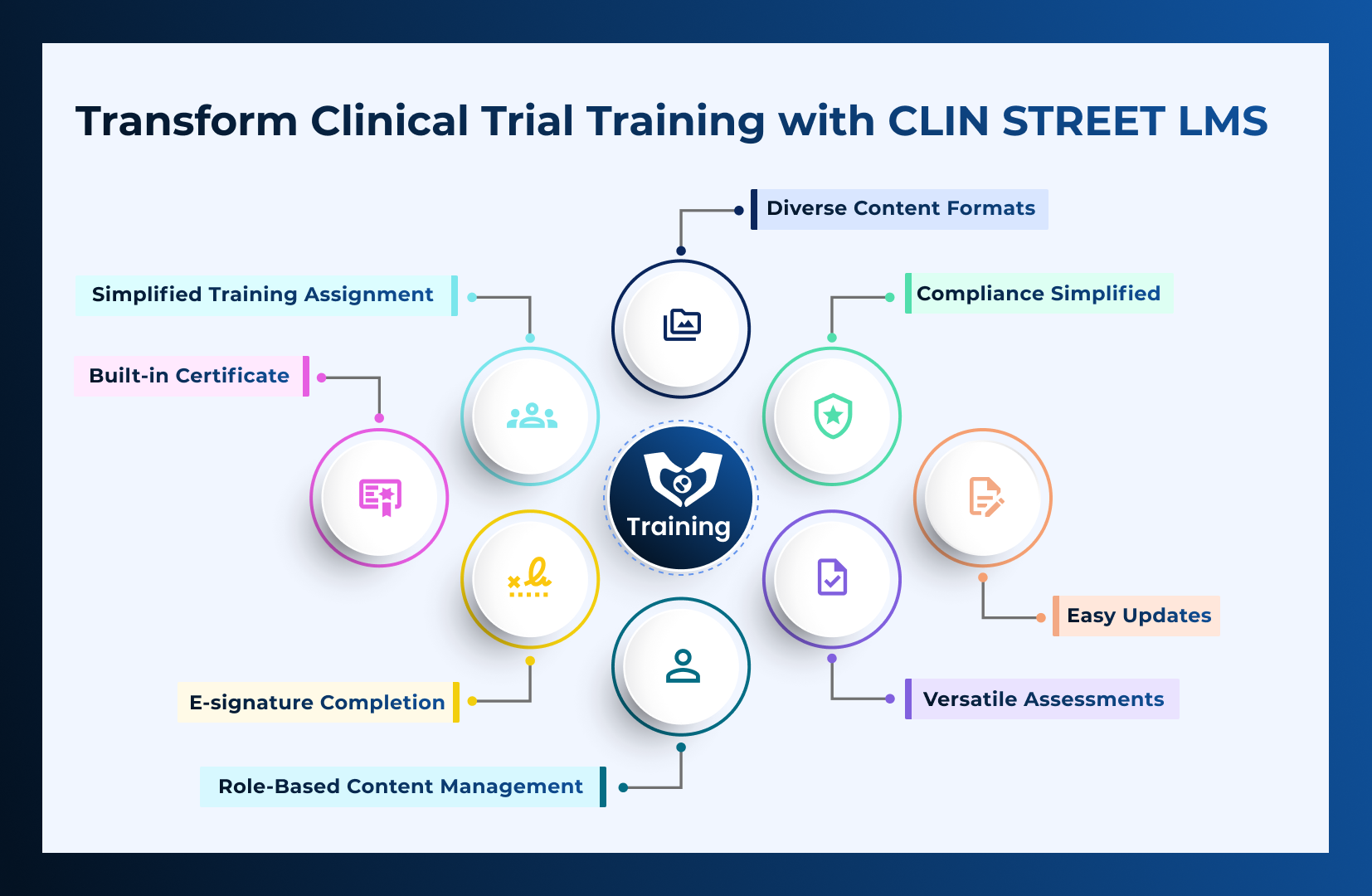
CLIN STREET LMS redefines training! It’s sleek, efficient, and packed with features to make administration a breeze. With a user-friendly interface and powerful tools,
Read More →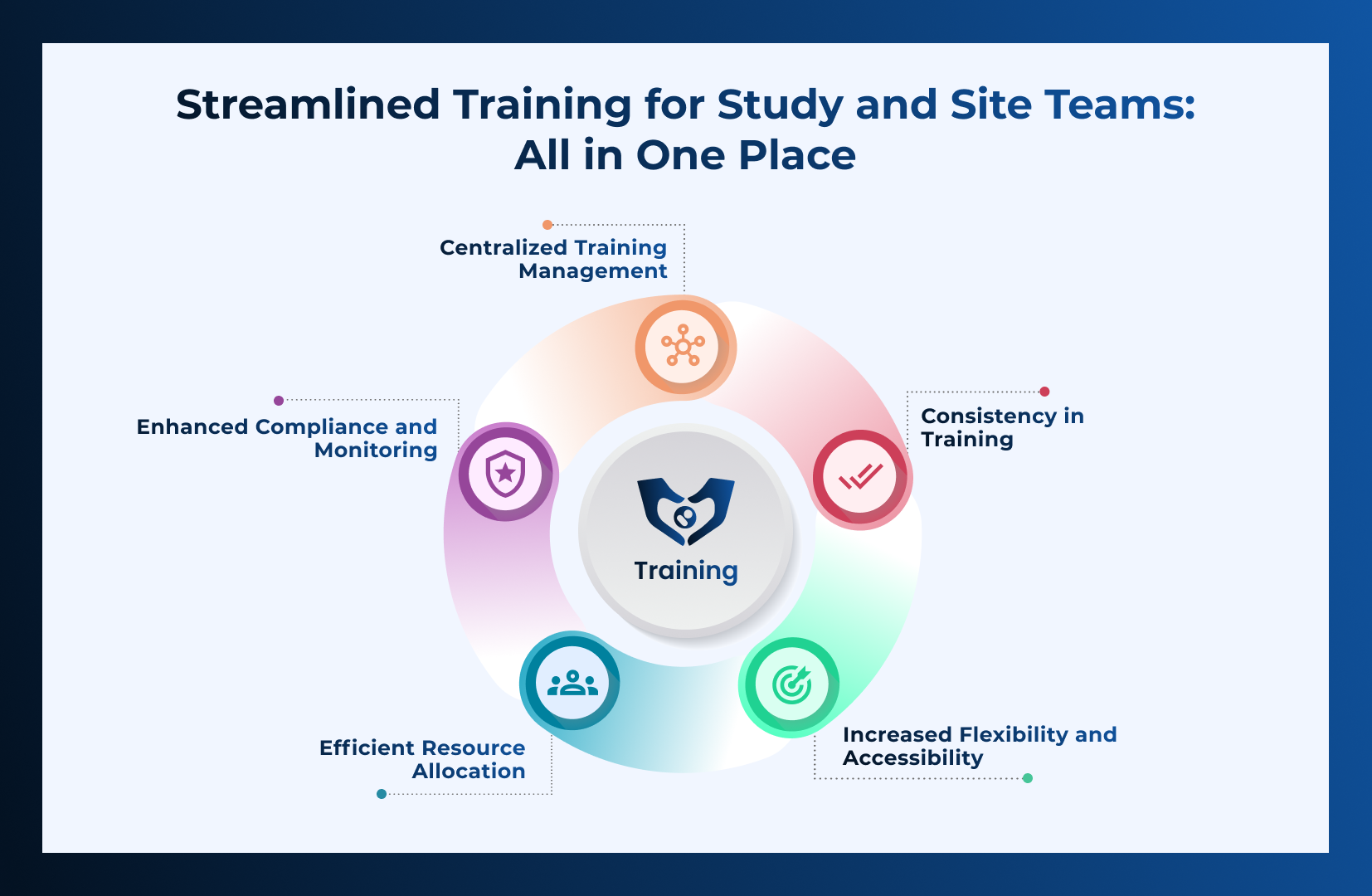
Compliance with regulatory requirements is paramount, in the world of Clinical Resarch. Organizations must adhere to guidelines set by bodies such as the US
Read More →