eReg
Key Features

Get started in minutes

Auto-File Documents
Optimize your workflow with pre-filled documents and automated filing, freeing up precious time for other tasks

Manage
Built-in document management
and reporting capability allows
you to pro-actively create and
assign tasks, due dates

Be Inspection Ready
With built-in management
features and quality-by-design,
always be inspection ready so you
never have to get ready
"Always be Inspection ready"
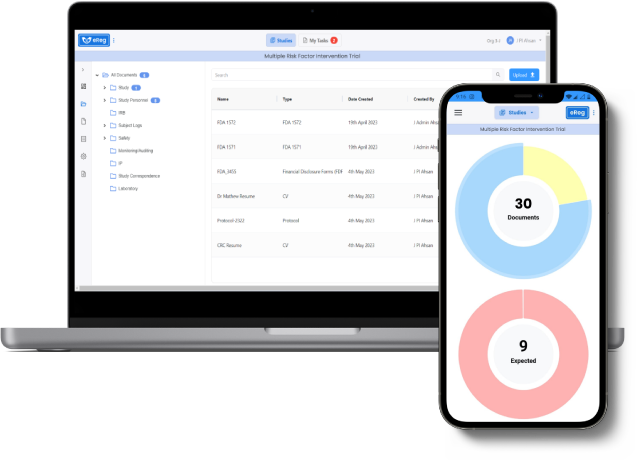
Introducing Clin Street’s eReg – the all-in-one regulatory binder solution for clinical trials. With eReg, managing regulatory documents has never been easier. This customizable solution streamlines the process of filing and storing documents, while also providing users with a built-in management tool to manage tasks related to these documents.
Benefits
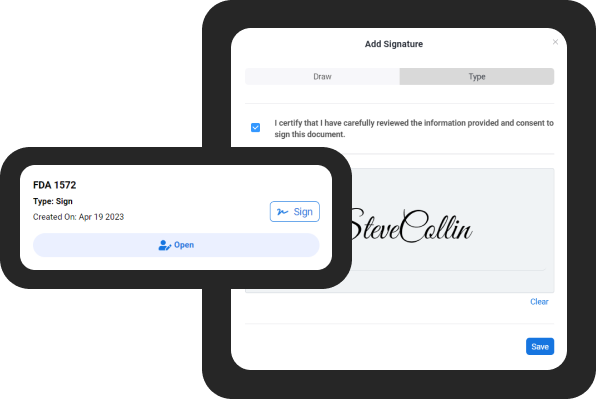
Get documents signed faster
- Sign documents remotely with e-signature feature
- Eliminate errors associated with paper-based signin
- Safeguard document security
- Embrace a streamlined signing process
Logs – Compliance by design
- Electronic logs for seamless log management.
- Ensure regulatory compliance effortlessly.
- Wide range of logs for comprehensive management.
- Go digital for fewer errors and improved productivity.
- Save time and streamline clinical trial processes.
Logs – Compliance by design
- Electronic logs for seamless log management.
- Ensure regulatory compliance effortlessly.
- Wide range of logs for comprehensive management.
- Go digital for fewer errors and improved productivity.
- Save time and streamline clinical trial processes.

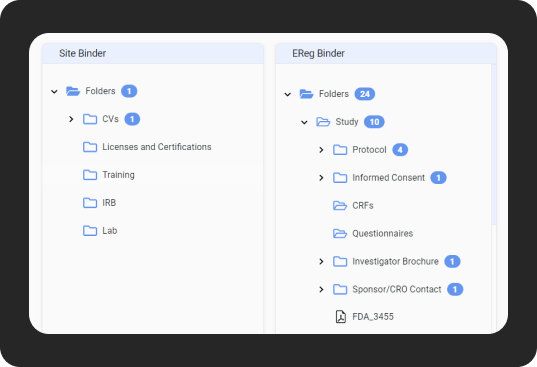
Auto-filing of documents
- Save time with auto-population and auto-filing.
- Eliminate redundant tasks.
- Reduce the risk of errors.
- Embrace seamless document management.
- Optimize document collection.
Remote Monitoring
- Enable remote inspection for auditors and monitors.
- Access documents anytime, on any device.
- Enhance efficiency and convenience.
- Securely access and review documents.
- Offers flexibility for different work environments.
Remote Monitoring
- Enable remote inspection for auditors and monitors.
- Access documents anytime, on any device.
- Enhance efficiency and convenience.
- Securely access and review documents.
- Offers flexibility for different work environments.

Security and Privacy










