The Trial Master File (TMF) is a critical component in clinical trials, serving as the repository for all essential documents. TMF demonstrate the compliance of the sponsor and investigator with Good Clinical Practice (GCP) standards and regulatory requirements. The TMF Manager plays a pivotal role in ensuring that the TMF is inspection-ready at all times. This blog explores the responsibilities of a TMF Manager in achieving inspection readiness, from the initiation of a clinical trial to its closure, while highlighting relevant regulatory requirements and recent inspection observations by major regulatory bodies like the FDA, EMA, and MHRA.
Regulatory Requirements for TMF:
Regulatory authorities such as the US FDA, EMA, and MHRA have established comprehensive guidelines for the maintenance and inspection readiness of the TMF. Key regulations include:
- FDA: 21 CFR Part 312.62 and Part 312.68 mandate the maintenance of accurate and complete records and their availability for inspection.
- EMA: Directive 2005/28/EC outlines principles and detailed guidelines for GCP in relation to investigational medicinal products.
- MHRA: GCP guidelines require that all trial-related documents be readily available for inspection.
Observations on Lack of Inspection Readiness:
Recent inspection data from major regulatory bodies highlight common issues related to TMF management:
- US FDA: In the last three years, there were 250 observations related to TMF deficiencies, with common issues being incomplete documents and poor document traceability.
- EMA: Reported 180 observations, frequently citing lack of timely updates and inadequate version control.
- MHRA: Noted 210 observations, often related to poor document organization and delayed document retrieval.
The Role of the TMF Manager:
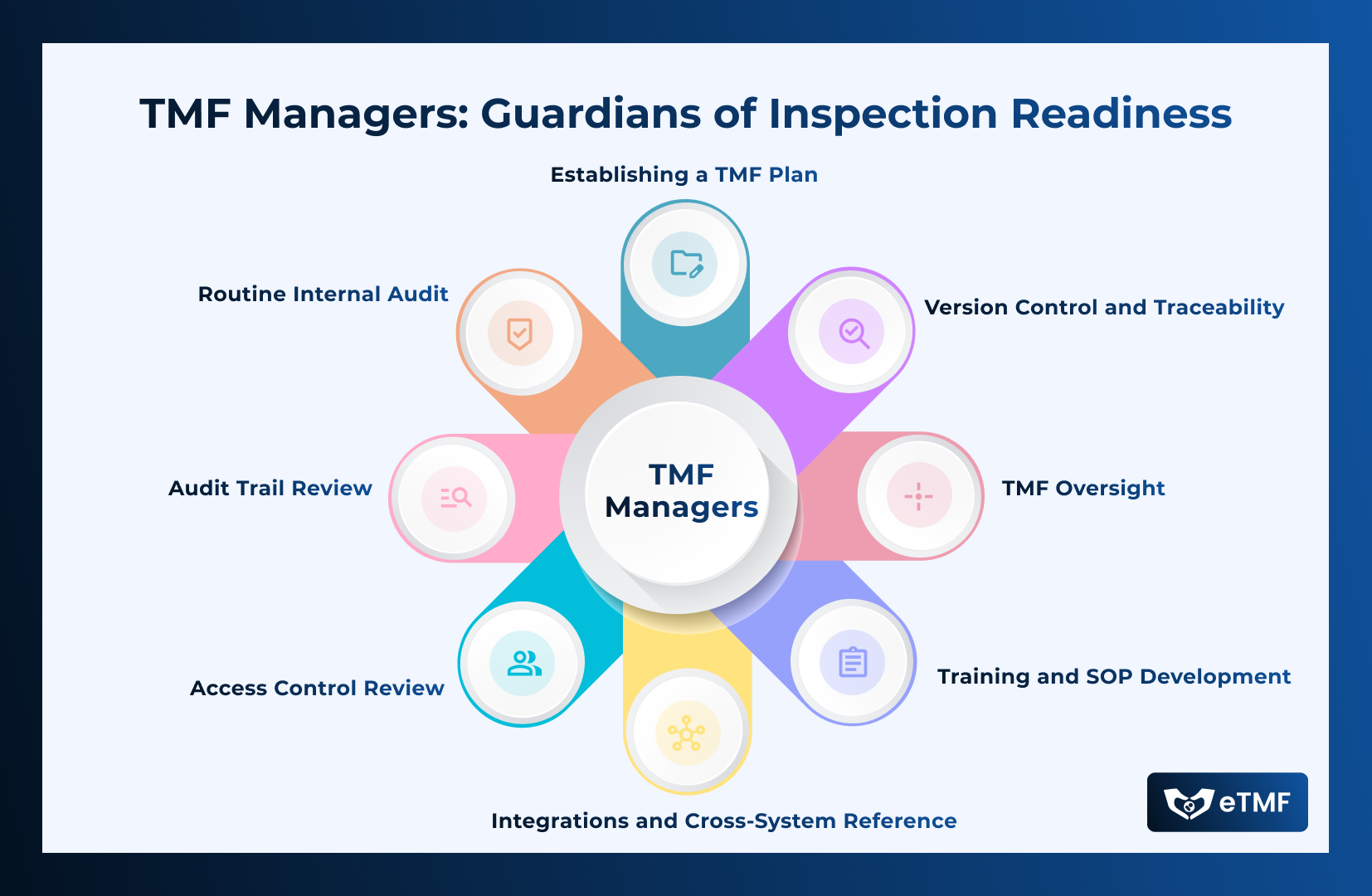
The TMF Manager plays an essential role in ensuring that the TMF is always inspection-ready, thereby supporting regulatory compliance and safeguarding the integrity of clinical trials. By adhering to regulatory requirements and implementing best practices, TMF Managers can significantly reduce the risk of non-compliance and enhance the efficiency of clinical trial operations. Here are some best practices for TMF Manager to drive the Inspection Readiness :
- Establishing a TMF Plan: Develop a comprehensive TMF Plan that details document management processes, roles, and responsibilities. Ensure the plan covers all workflow stages of the TMF documents, specifying clear expectations for each stakeholder at every stage. Include ongoing oversight, risk assessment, and compliance evaluation to drive adherence to standards.
- Training and SOP Development: Ensure all team members are trained on TMF processes. Document every TMF management process under Standard Operating Procedures (SOPs) for consistent application.
- TMF Oversight: Oversee the timely collection, review, and filing of all essential documents within the eTMF. Establish a process to monitor study performance using predefined Key Performance Indicators (KPIs) such as Completeness, Quality, and Timeliness. Use KPIs to identify gaps and implement improvements.
- Version Control and Traceability: Implement robust version control to maintain the integrity of the TMF, ensuring all changes are traceable.
- Integrations and Cross-System Reference: Integrate with eClinical solutions of other stakeholders to enhance eTMF compliance. Focus on transferring documents across systems, gathering insights from triggers, and obtaining data for reconciliation. If integration is not possible, manually gather data to perform reconciliation and proactively identify TMF gaps.
- Access Control Review: Regularly review access privileges for active users to ensure the highest level of eTMF integrity. Keep access controls current and relevant for each user.
- Audit Trail Review: Recognize that not all gaps or potential observations stem from document content or filing. Thoroughly evaluate audit trails to reconstruct events and ensure there are no risks or gaps affecting the trial’s reconstruction and TMF integrity.
- Routine Internal Audit and Continuous Improvement: Be proactive. Establish a process for conducting internal audits with the same rigor as regulatory inspections. Act promptly on identified gaps and implement process improvements to enhance TMF compliance.
Maintaining a well-organized and complete TMF is not just a regulatory necessity but also a cornerstone of successful clinical trial management. Through diligent planning, continuous monitoring, and proactive preparation, TMF Managers can ensure their organization is always ready for inspection.
CLIN STREET’s eTMF platform offers a comprehensive suite of features designed to ensure best in class management of TMF towards achieving Inspection readiness. Key features include:
- Built-In Dashboard : Real-Time Comprehensive dashboards aiding oversight covering all KPIs such as Completeness, Quality, Timeliness.
- Site Level -Dashboards: Visibility of Site level expected documents per site in dashboard view along with oversight on progress of document filing per milestones and events.
- Real-Time Built-In Report : In-depth visibility on reports to assess gaps and derive triggers for improvements
- AutoPilot : First class Built-In automation that elevates the management of milestones and events and drive Trials towards compliance.
- Milestone-Event driven expected document creation : Best in class feature facilitating creation of expected documents in trial, while associating those with milestones and events.
- Milestone-Event Based Document Management: Efficiently collect and assess document completeness in alignment with critical milestones and events, ensuring timely and organized document handling.
- Comprehensive Document Tracking: Seamlessly plan, manage, and monitor all expected documents within the eTMF, ensuring nothing falls through the cracks.
- Direct Site Upload Capability: Simplify the document submission process with direct upload capabilities for sites, enhancing efficiency and reducing delays.
- Integrated Reporting and Notifications: Stay informed with built-in reports and automated notifications, keeping you up-to-date on TMF status and facilitating prompt action when necessary
- Comprehensive Audit Trail: In-detail audit trail giving visibility of each task performed within TMF per user.
- Integration with LMS: Configurable integration with Clin Street’s LMS for auto filing of Training certificate, driving compliance of the TMF
Driving Inspection Readiness for TMF can be complex, and we understand your unique needs. That’s why we’re here to introduce you to our CLIN STREET eTMF platform, designed to simplify your clinical trial management. Let’s collaborate to tailor our solutions precisely to fit your project’s requirements by scheduling a personalized demonstration here. Alternatively, you can also contact us at hello@clinstreet.com. Our team is available around the clock to accommodate your schedule and provide the support you need.
About the Author: Shounak Damle is a seasoned clinical trial professional with extensive experience in TMF management and other eClinical technologies. With a passion for quality assurance and compliance.